Beyond the Pill: Expanding pharma’s patient funnel with digital dx tools
I recently had a fascinating conversation with a pharmaceutical program head about their investment in digital diagnostic tools. To me, this represents a significant shift - pharma companies are no longer just manufacturing drugs and waiting for providers to find suitable patients. Instead, they're creating tools to identify potential patients earlier.
This dual-purpose approach helps patients get diagnosed sooner (improving outcomes) while also expanding the company's addressable market. By building these diagnostic tools, pharma is creating their own patient funnels. It’s the “beyond the pill” paradigm shift where pharma is positioning itself as an integrated healthcare solutions provider rather than just a drug manufacturer.
I wanted to learn more about this problem so I took an hour or two and jotted down some thoughts. It helped me organize the problem into the standard framework I used at GRAIL (FDA’s AI Lifecycle). Enjoy!
Problem: [company] wants to integrate a new diagnostic digital tool into healthcare systems
Outcome: Improve patient outcomes by reducing time from symptoms to diagnosis
Ideal workflow: Doctor opens patient chart → Sees alert suggesting "This patient shows early indicators of [disease]" → Can click for details or order recommended tests with one click → Patient diagnosed early → Doctor takes [company]’s desired action
Assumptions
[company] has integrated with healthcare systems for post-diagnostic workflows
[company] will use one disease for its MVP and first FDA approval
[company] is beyond the data sourcing step (e.g. has technical access and governance infrastructure in place to acquire data for early R&D before formal clinical trials)
[company] has a cybersecurity and regulatory strategy established for integrations and FDA submissions therefore I have scoped these out of the strategy
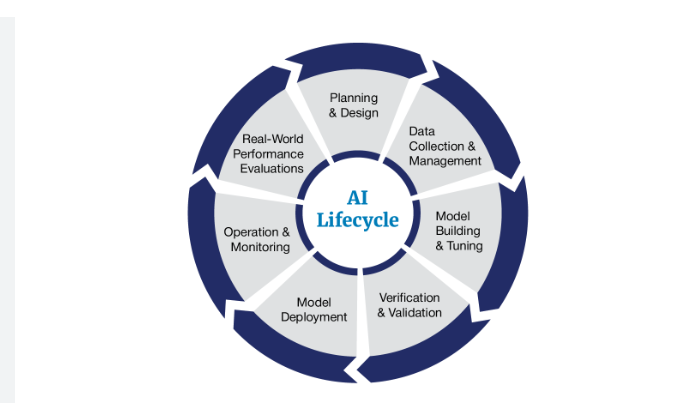
[company] will follow the FDA’s AI product lifecycle framework summarized below (this is what I used at GRAIL)
FDA’s AI Lifecycle
Approach
Planning & Design
Choose one disease modality for the MVP
Define data strategy for representativeness of patient population
Conduct competitive analysis and research to see what other companies have integrated diagnostic tools into pre-diagnosis workflows. Research should be broad and include everything from wearables (e.g. Apple watch) to medical devices.
Conduct early experiments to define minimum viable performance criteria for MVP algorithm (e.g. the accuracy threshold below which the algorithm isn’t clinically valuable)
Define appropriate primary and secondary endpoints for early R&D and future clinical trials
Identify physicians or health systems that can be influential early adopters where [company] already has post-diagnosis integrations. Document clinical workflows as user stories.
Data Collection and Management
Training & Validation: Algorithm needs sufficient RWD for training and validation on disease preconditions that’s representative of the target population.
[company] leverages data sourcing infrastructure and regulatory framework to acquire datasets for early development
Model Building and Tuning
Host “bake offs” within data science teams for different models to identify those with peak performance. GRAIL hosted multiple bake-offs to create algorithms with different specificity and sensitivity based on clinical use cases.
Create “fairness” eval to ensure the algorithm performs consistently across demographic
Verification and validation
Run RWD trials to measure primary and secondary endpoints
Test integrations with actual EHR systems in sandbox environments where [company] already has post-diagnostic integrations established
SaMD validation/verification documentation
Model Deployment
Use FHIR API. Couple of different options I’d explore:
Option 1: Direct EHR integration via app marketplaces
When a doctor is reviewing a chart, they can click on the [company] app icon within the EHR, which then analyzes patient data and shows potential pre-disease indicators
Option 2: Standalone web portal with EHR integration
Doctor is reviewing labs, clicks on a diagnostic assistant link, and is taken to [company]'s tool which has already pulled relevant patient data
Option 3: API integration with existing clinical decision support systems
When a doctor orders a lab tests, the system suggests disease screening tests based on [company]’s tool’s analysis of patient risk
Ideally implement a pilot with 1-2 early adoption champions
Operations and Monitoring
Create a continuous monitoring system to compare tool predictions to diagnoses
Create a mechanism for clinicians to report false positives/negatives (this was very important at GRAIL)
Real World Performance Evaluations
Track reduction in primary and secondary outcomes (e.g. time-to-diagnosis)
Collect physician satisfaction data on tool utility and workflow integration
Develop case studies highlighting successful implementations for future sales